Introduction
Creatine monohydrate, a staple in the supplement aisles of gyms worldwide, is celebrated for its ability to boost athletic performance, particularly in high-intensity, short-duration activities. But have you ever wondered how this powerhouse compound is made? Let’s delve into the fascinating process of manufacturing creatine monohydrate.
The Natural Path: Creatine in Nature
Before we dive into industrial processes, it’s worth noting that creatine exists naturally. Our bodies synthesize it from the amino acids glycine, arginine, and methionine, primarily in the liver, kidneys, and pancreas. This natural production is one way we get creatine, but it’s not enough for those looking to maximize their athletic performance, which is where supplements come in.
Industrial Synthesis: The Science of Creatine
1. Starting Materials:
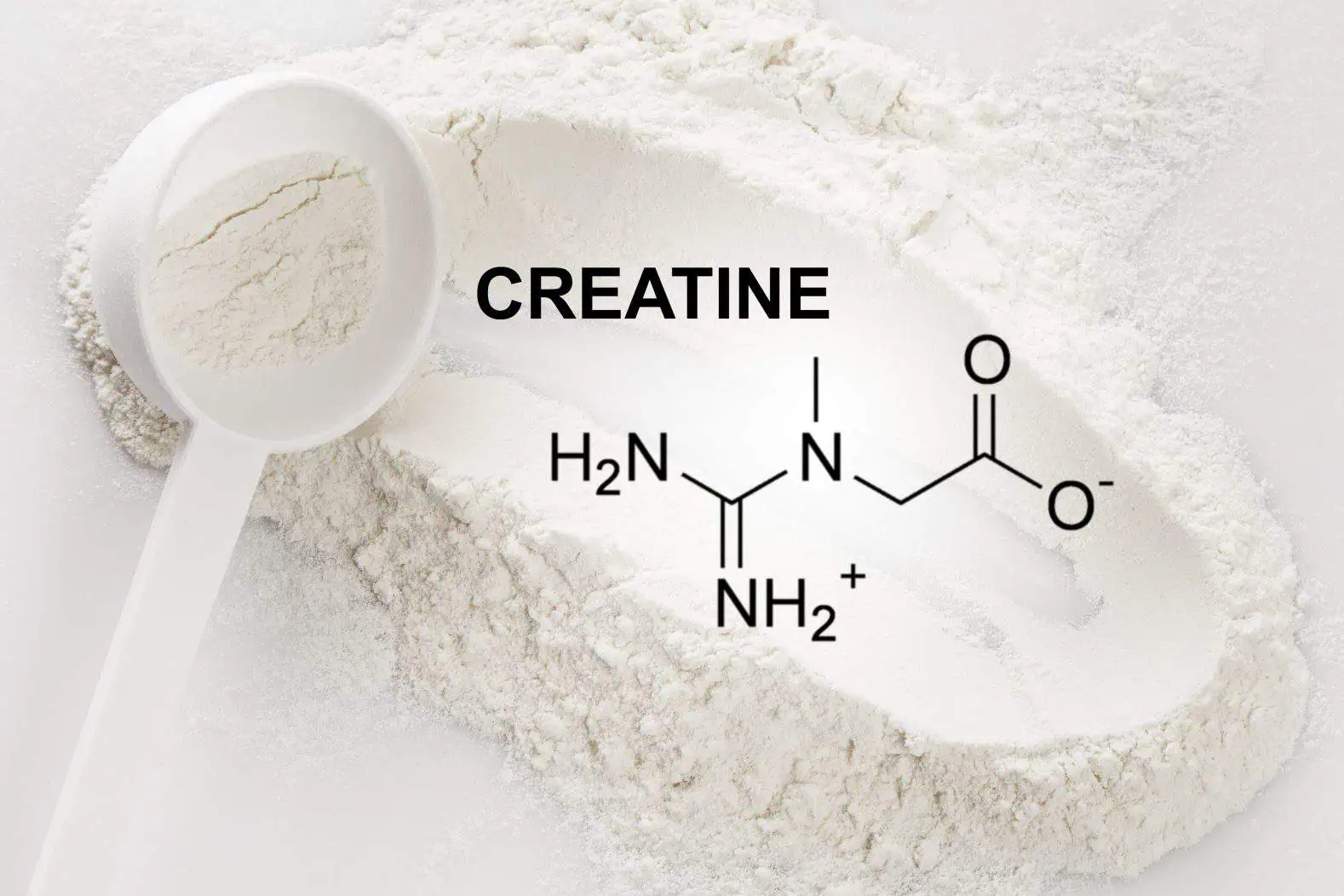
The synthesis of creatine monohydrate begins with two key ingredients: sarcosine (N-methylglycine) and cyanamide. These compounds are not just chosen at random; their chemical properties make them ideal for the reaction.
2. The Reaction:
In a controlled environment, sarcosine and cyanamide are mixed in the presence of a base, often sodium methoxide or sodium hydroxide. This reaction typically occurs in a solvent like methanol or ethanol to facilitate the process.
The reaction yields creatine, but it’s still in a raw, unrefined form.
3. Neutralization:
After the reaction, the mixture is neutralized to halt further chemical reactions, ensuring the creatine remains stable.
4. Purification:
Precipitation: Water or another anti-solvent is added to the reaction mixture, causing the creatine to precipitate out. This step is crucial for separating the creatine from other byproducts.
Crystallization: The mixture is often cooled or further treated to encourage the formation of creatine monohydrate crystals. These crystals are then filtered out.
5. Drying:
The wet creatine crystals are dried to remove any remaining solvents or water, ensuring the product’s stability and longevity.
6. Quality Control:
The dried creatine monohydrate undergoes rigorous testing. Techniques like High-Performance Liquid Chromatography (HPLC) are used to check for purity, ensuring that what you get is at least 99.9% creatine monohydrate, free from contaminants.
The Importance of Purity
Why is purity so crucial? Impurities can affect the effectiveness of creatine and potentially introduce health risks. High-quality creatine should be free from contaminants, ensuring that athletes and fitness enthusiasts get the benefits they expect without any unwanted side effects.
Conclusion: From Lab to Label
The journey of creatine monohydrate from a chemical reaction to your supplement bottle is a testament to modern chemistry’s ability to enhance human performance. Each step in the manufacturing process is designed to maximize purity and effectiveness, ensuring that when you reach for your creatine supplement, you’re getting a product that’s been meticulously crafted for optimal performance.
Understanding how creatine is made not only demystifies the supplement but also underscores the scientific rigor behind what might seem like a simple powder. Next time you take your creatine, you can appreciate the alchemy behind it, turning simple compounds into a powerhouse of athletic enhancement.




