Below is a fairly technical report of Curcumin. We suggest watching a helpful video entitled, “Only Take This Form of Curcumin” from Dr. Chad Larson below that breaks it all down rather nicely.
Turmeric is one of the most revered plants in Ayurveda, the Indian traditional medicinal system. Commonly identified as “yellow curry powder” in western culture, Turmeric originated in the Indian subcontinent where it was used as a spice, food colorant, food preservative and as a medicinal herb.
In fact the ancient Ayurvedic texts written almost 2,500 years ago, describe the benefits of Turmeric rhizomes for its use in treatment for rheumatism, asthma, various allergies, liver disorders, poor appetite and influenza.
Science on turmeric greatly advanced with isolation and identification of its active principles, Curcuminoids. These curcuminoids were found to be responsible for not only the yellow color of turmeric roots but also for the several health benefits for which turmeric has been used for centuries.
Today the active components of turmeric, collectively known as curcuminoids, are among the most promising natural compounds studied across the globe. Clinical studies have revealed their active role in management of several chronic health conditions. Several pioneering studies have been conducted by major Universities, research institutes and hospitals on Curcumin C3 Complex® which form the platform for its use in many health conditions. These studies also helped our understanding on the metabolism of Curcumin inside the body.
Curcuminoids provide a very interesting concept of a pronutrient. On ingestion, the Curcumin forms several active metabolites undergoing phase I metabolism such as dihydrocurcumin, tetrahydrocurcumin, hexahydrocurcumin and octahydrocurcumin. In course of its metabolism inside the body it also forms degradation compounds such as ferulic acid and bicyclopentadione. While these phase I metabolites have shown to have beneficial biological activity, compounds such as curcumin glucuronides and sulfates formed after phase II metabolism have shown to be ineffective in independent studies.

CURCUMINOIDS & NF-kappaB
Nf-kappaB (NfkB) is a transcription factor that is a master regulator of inflammation – it triggers a cascade of inflammatory reactions through several cytokines. NfkB is a major mediator in a wide variety of chronic diseases ranging from cancer to cardiovascular diseases 1-3. The Curcuminoids found in turmeric inhibit NfkB thus alleviating the effects of inflammation. They also support a healthy immune system, provide antioxidant protection, protect cell and organelle membranes from lipid peroxidation, and inhibit destructive enzymes in joints and tissues.
With Curcumin bioavailability still discussed at length, and with various formulations making variety of claims on the bioavailability (X times or XX times more than nearest competitor), the consumers are getting confused. The purpose of this article is to help the formulators, clinicians, marketing companies and consumers to make well informed choices when it comes to Curcumin formulations for their health.
CURCUMIN AS A PRONUTRIENT
Extensive reviews have been written on Turmeric and Curcumin 4,5. While the biological activity of Curcumin has been well established, in clinical set up, the detection of low amount of Curcumin in the blood after acute administration and in dose escalation study has lead to a feverish debate on the metabolic fate of Curcumin 6.
With the purported aim of marketing a Curcumin product that can provide the improved absorption of Curcumin in the body and thus its content in blood led the way to a variety of formulations in the market. We will discuss these formulations later in this article.
Meanwhile the previous clinical studies on Curcumin revealed that though the Curcumin itself was found to be low in blood, most of circulating Curcumin was detectable in conjugate forms such as glucuronide and sulfate forms, the levels found in the blood serum ranged between 22 to 41 ng/ml 7.
These important findings suggest rapid biotransformation of curcumin. Further the studies conducted on the phase II conjugates of curcuminoids showed that conjugates such as sulfates and glucuronides have very poor biological activity.
In order to understand the potential of phase II metabolites of curcuminoids, a group of researchers synthesized and studied the biological activity of these phase II metabolites against human cell lines and directly compared it with curcumin. These extensive studies proved that the phase II Curcumin glucuronides did not exhibit any significant biological activity which are normally attributed to the three curcuminoids 8.
While the Curcumin glucuronides did not show any biological activity, in a path- breaking study published in PNAS, in 2011, Hassaninasab et al. demonstrated that Curcuminoids undergo immediate biotransformation by NADPH dependent Curcumin/Dihdrocurcumin reductase into most biologically active tetrahydrocurcumin, a phase I metabolite 9.
Previous studies have shown that Tetrahydrocurcumin, Hexahydrocurcumin and Ferulic acid all have exhibited potent biological activities 10,12. Hexahydrocurcumin has been studied and found to have antifungal, anticancer, antioxidant and human platelet aggregation properties (Figures 1 and 2) 13-16.
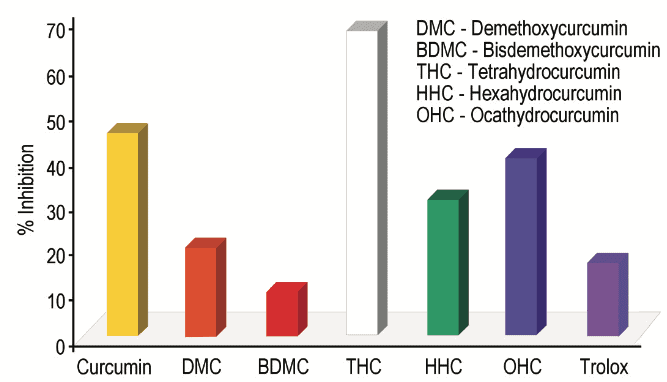
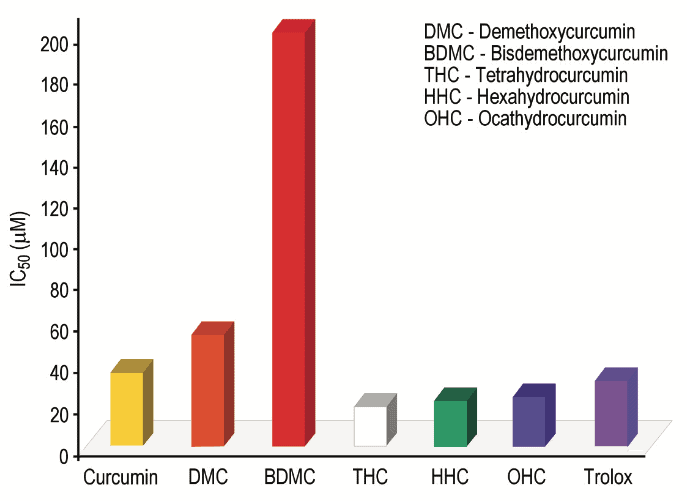
These findings strengthen our concept of Curcumin as a pronutrient. While Curcumin itself has strong potential activity it exhibits its biological activity through selective metabolites. Therefore the conversion pathways or biological transformation of Curcumin inside the body are important (Figure 3).
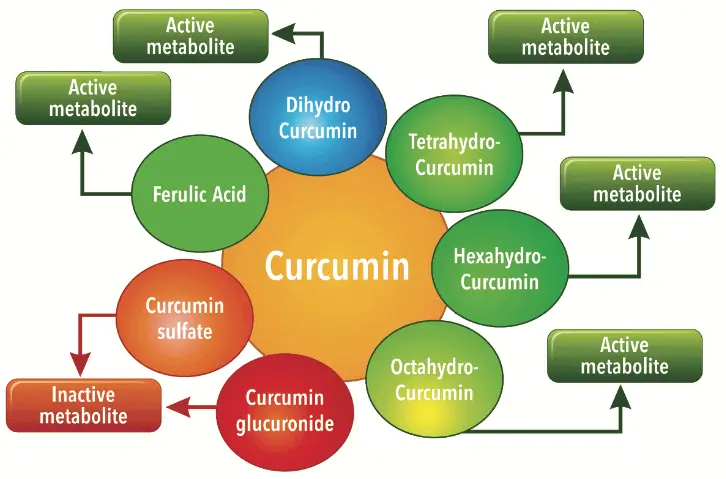
EVALUATING BIO ENHANCEMENT TECHNIQUES AND MECHANISM OF THE ACTION
As we now understand that while increasing bioavailability of curcuminoids, we cannot neglect or ignore the pharmacological roles of its active metabolites. Hence increasing the bioavailability or presence of phase II conjugates cannot produce any beneficial effect of Curcumin since these phase II conjugates do not have biological activity, whereas what we need to increase is the unconjugated form of curcuminoids. So the question, which we need to answer, is whether the current techniques used in the claimed bioenhanced Curcumin preparation are capable of providing the unconjugated Curcumin to the body or they are merely increasing the biologically inactive phase II conjugates of curcuminoids. As we will study below some of the bioenhanced Curcumin and techniques used for making Curcumin more bioavailable, we will also compare the safety of dosage, highest dosage studied, clinical studies, and content of curcuminoids in the formulations.
1. BCM 95® – Curcumin Formulated with Turmeric Oil
An example of this approach in market is BCM- 95®/BIOCURCUMAXTM, a “Curcuminoids Complex with Volatile compounds not less than 95%”. The product was patented in 2002 and the patent method discusses increasing the bioavailability of 98% pure Curcumin from a presumptive benchmark levels of 60-65% to 90-97% levels with added turmerone- enriched oil.
The presumption the marketers make in their bioavailability claim for Curcumin is based on the measurement of Curcumin in an unscientific approach due to the spectroscopic method used to estimate unabsorbed Curcumin in the fecal matter. Another anomaly in the study mentioned in the patent actually mentions range of absorption of 60-65% for unmodified Curcumin, which would be considered an extremely bioavailable compound by any standards, therefore this measurement in the bioavailability context, casts a long shadow of doubt on the protocol and analytical methodology adopted in the study which was conducted to prove the bioavailability of BCM-95® only.
Turmeric essential oil has not been proven to be safe for humans, and one study by Prof. Janet Funk clearly showed that Tumeric oil has a detrimental effect on the protective actions of Curcuminoids 18.
2. Meriva® – Curcumin Formulated with Phosphatidylcholine
A second formulation consisting of Curcumin (20%), Phosphatidylcholine (40%) and microcrystalline cellulose has been reported to increase the bioavailability of Curcuminoids 29 times 19.
However, what the product actually does is increase the concentration of the phase II metabolic products, namely the glucuronides and sulfates, which are the inactive forms of Curcumin. These glucuronides, as recounted earlier, neither display activity against cancer cells nor inhibit pro-inflammatory NFkB. Their bioavailability claim is based on a single human study involving only nine subjects divided in three arms of the study (three subjects each).
Studies on this brand of ‘enhanced’ Curcumin only substantiate the known anti-inflammatory effects of curcuminoids, but do not show or prove the benefits of any enhanced bioavailability on their existing results. The studies conducted only demonstrate the end points which were achieved by previous studies using Curcuminoids alone.
A new report on phosphatidylcholine appeared in the Nature News and Views article published in the columnCardiovascular Disease20 that reviewed the evidence high-fat foods are rich in the lipid phosphatidylcholine – the ingredient used in Meriva® – and its metabolite choline. Intestinal bacteria convert phosphatidylcholine and choline into Trimethylamine oxide (TMAO). The liver enzyme FMO3 converts Trimethylamine (TMA) into TMAO – a metabolite that is then found in the blood. Research conducted by Wang et al. as published in Nature21 showed that circulating levels of TMAO may contribute to greater plaque formation in the arteries, and could result in heart disease.
3. CurcuWIN™ – Curcumin Formulated with UltraSOL™ Technology
The manufacturer of a product called CurcuWIN™ claims 46 times greater Curcumin bioavailability compared to standard Curcumin, BCM-95® or phytosomal Curcumin, Meriva®. The product claims to use an unknown ‘UltraSOLTM nutrient technology’, which increases all of the pharmacologically inactive Curcumin conjugates, including the glucuronides and sulfates.
4. Theracurmin® – Curcumin Using Nanotechnology
An ‘enhanced’ Curcumin product called Theracurmin® using a nanotechnology and water dispersion technology claims 40 times greater bioavailability in rats and 27 times higher bioavailability in humans compared to powdered Curcumin 22.
This technology also increases all of the pharmacologically inactive Curcumin conjugates, namely the glucuronides and sulfates.
5. Longvida® – Curcumin Formulated into Solid Lipid Particles Using Lecithin
Longvida® is marketed as “solid lipid particle formulation of curcumin”23. The manufacturer claims that their technology increases the bioavailability of Curcumin 65 times and states that their Curcumin detected in blood serum is free Curcumin and not glucuronidated or conjugated Curcumin metabolites. However no detailed toxicity studies are available on such products of recent origin. Here again lecithin could produce TMAO, a cardiotoxic chemical.
6. CAVAMAX® W8 – Curcumin Formulated with Cyclodextrin
CAVAMAX® W8 is an ‘enhanced’ Curcumin made by complexing Curcumin with gamma cyclodextrin. The in vivobioavailability was measured in rats and showed a 10 to 20 times higher total curcuminoids. According to their research “Animals that received CAVAMAX® W8 curcumin showed a 10 to 20 times higher amount of total curcuminoids in their blood plasma, expressed as the sum of free curcumin and its metabolites (curcumin sulfates and curcumin glucuronides), than animals that received a commercial product or pure curcumin powder. This huge difference in HPLC- measured curcumin metabolites indicates that a maximum amount of curcumin was delivered into the blood stream of the rats, which can only be explained by the very highly bioavailable CAVAMAX® “W8 curcumin.”
In a human serum pharmacokinetic study, the manufacturer concluded that “Curcumin uptake was at least 4.4 times higher than the next-best commercial curcumin formulation in this clinical study.” The problem with these measurements in the rat and human study is that they estimate inactive metabolites, assuming that the metabolites are active, which they are not.
Cyclodextrin complexes are often used to increase the bioavailability of natural compounds. However, there is a toxicity issue where some cyclodextrins solubilize cholesterol, which can then be depleted from cell membranes. Beta cyclodextrin has a regulatory limit of 5 mg/kg/day because it is known to deplete cholesterol from cell membranes in this manner24.
Another toxicity issue has been revealed in rat studies where cyclodextrins were thought to increase the solubility and absorption of toxic contaminants in ingested food 25.
7. Curcumin Formulated with BioPerine®
This combination consist of two GRAS, patented ingredients Curcumin C3 Complex® and BioPerine®, a Piper nigrumextract. BioPerine® has been clinically studied for increasing the bioavailability of several nutrients including Curcuminoids. In a clinical study conducted in 1998 on healthy volunteers, it was found that BioPerine® can increase the bioavailability of curcuminoids by 20 folds26. This increase was measured for the Curcumin itself and not for its phase II metabolites. One of the reasons for this finding comes from the fact that the BioPerine® consists of piperine which is known to inhibit the formation of phase II metabolites because of glucuronidation. Piperine reduces glucuronidation by inhibition of UDP- glucuronyl transferase activity and by lowering the levels of endogenous glucuronic acid. Curcumin has tendency to undergo rapid biotransformation, its conversion in particular to Phase II metabolism is often seen as detrimental to bioactivity. BioPerine® helps in inhibiting the formation of phase II metabolites of Curcumin27. Piperine promotes nature’s own mechanism namely the flippase activity by P-gp inhibition and reduces the glucoronidation of Curcumin. This effect is short lived and requires simultaneous consumption of 5 mg of BioPerine® along with Curcuminoids. BioPerine® does not indiscriminatingly increase the Curcumin bioavailability. Other formulations increase the glucoronidation of Curcumin. In addition piperine has thermogenesis activity. As the thermogenesis occurs, the demand of fresh nutrients increases to sustain the metabolic process, facilitating better absorption of nutrients in intestine.

Safety of Curcumin C3 Complex® and BioPerine® has been evaluated in a clinical study performed by Tufts University. The results of the study showed that Curcumin and BioPerine® combination can be safely taken without any interaction with commonly prescribed medication such as flurbiprofen, acetaminophen and midazolam28. However no such data on drug interactions is available with any other curcumin formulation.
Extensive biotransformation in the body, an in-depth knowledge of Curcumin biotransformation and role of metabolites inside the body is important to apply the concept of bioavailability more constructively.
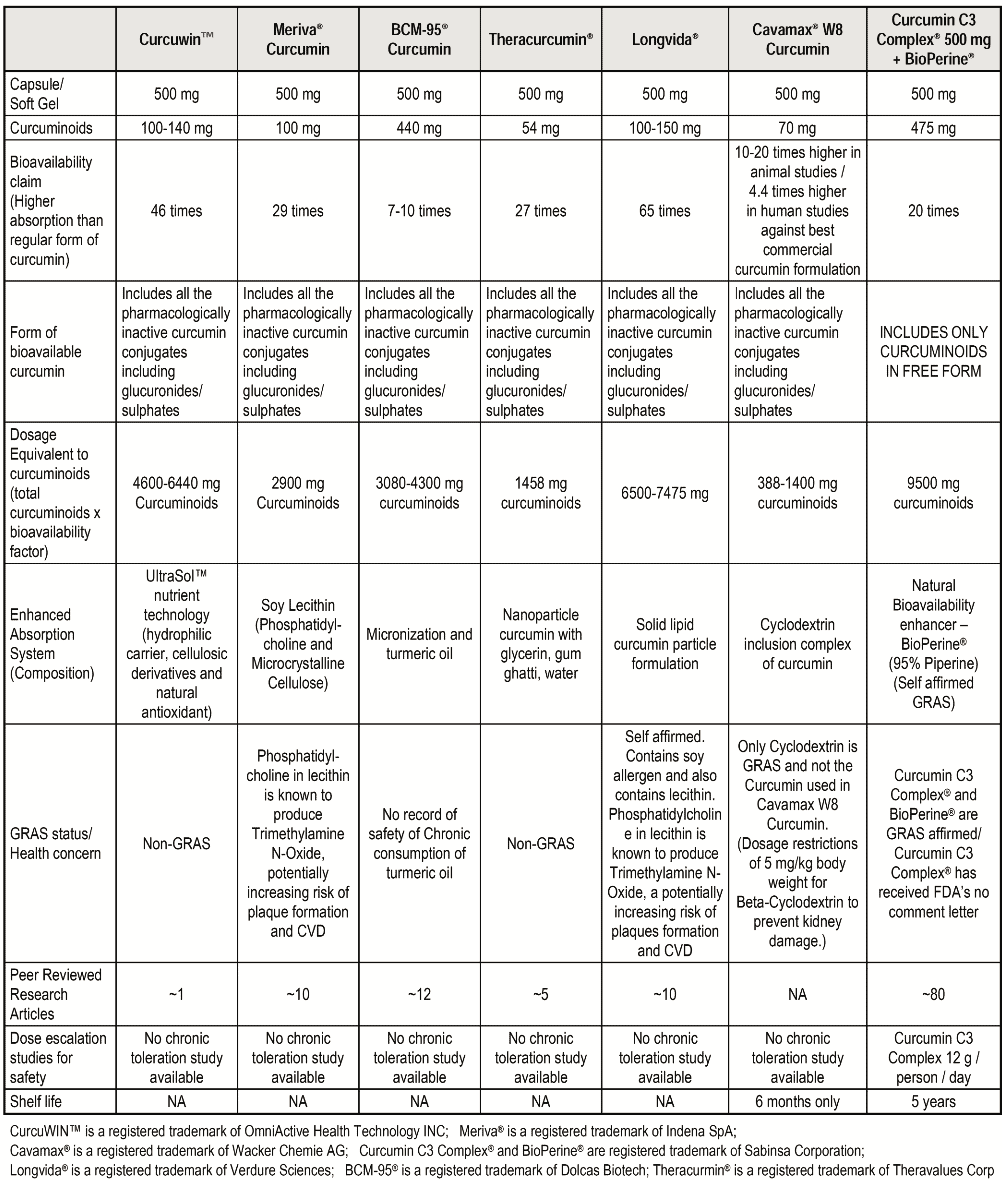
Table I gives an interesting comparison of above seven curcumin preparations in context of their composition, bioavailability, actual Curcumin in the product as well as estimated bioavailable Curcumin in the body.
The chart is informative for retailers & consumers to understand the difference between various curcumin preparations available in the market. The table can assist in choosing the right formulation by providing answers to following questions:
– Which formulation has maximum Curcumin in the product based on both composition as well as dose equivalency (Available Curcumin= Bioavailability Factor X content of Curcumin)
– What forms of Curcumin are made bioavailable (Does it include phase II metabolites?)
– Which formulation is GRAS and safe
– Use of excipients and associated toxicity
– How many clinical studies have been conducted on the formulations
CONCLUSION
Discussion on curcumin’s bioavailability is not complete without discussion on its metabolites. All the metabolites of Curcumin are not alike and hence increasing the bioavailability of phase II metabolites is not of any biological significance as we see in many of the formulations discussed above. We also see shrinking dosage of the Curcumin advised by many formulators based on higher – amount of presumed bioavailability. This is not a healthy approach as these formulations are merely increasing the biologically insignificant phase II metabolites rather than increasing the active metabolites or Curcumin itself.
Bioavailability is an important aspect of nutrient metabolism and its concept should not be used for just marketing purpose. Indiscriminately increasing bioavailability of Curcumin is also not going to bring the best out of Curcumin. Infact increasing bioavailability beyond certain level can have harmful effects such as QT prolongation and haemolysis. Increased bioavailability of curcumin has implications in QT prolongation by hERG channel inhibition. This has been pointed out by recent work29. Lipophilic Curcumin formulations have been developed and tested for safety. One concern is that the tissue-specific buildup of Curcumin conjugates can lead to toxicity. Preclinical research in beagle dogs with a liposomal Curcumin product demonstrated a dose-dependent hemolysis believed to be caused by a build-up of Curcumin metabolites, in particular Curcumin glucuronides and conjugates in tissues. The researchers in this study developed a method to help prevent this from occurring30.
Today we know about the curcumin’s metabolic fate much better and this knowledge on its metabolites should be considered in any discussion on bioavailability to have a constructive approach on improving curcumin’s functionality.
references
1) Ganjali S, Sahebkar A, Mahdipour E, Jamialahmadi K, Torabi S, Akhlaghi S, Ferns G, Parizadeh SM, Ghayour-Mobarhan M. The Scientific World Journal 2014; doi 10.1155/2014/898361
2) Chuengsamarn S, Rattanamongkolgul S,Phonrat B, Tungtrongchitr R, Jirawatnotai S. Nutr. Biochem. 2014, 25 (2),144-50.
3) Khajehdehi P, Pakfetrat M, Javidnia K, Azad F, Malekmakan L, Nasab MH, Dehghanzadeh G. Scand. J. Urol. Nephrol. 2011, 45 (5), 365-70.
4) Nagpal M. Sood S. J. Nat. Sci. Biol. Med. 2013, 4 (1), 3-7.
5) Curcuma longa (turmeric). Monograph. Altern. Med. Rev. 2001, 6 Suppl, S62-6.
6) Lao CD, Ruffin MT 4th, Normolle D, HeathDD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL, Brenner DE. BMCComplement. Altern. Med. 2006, 6, 10.
7) Dhillon N, Aggarwal BB, Newman RA, WolfRA, Kunnumakkara AB, Abbruzzese JL, N CS, Badmaev V, Kurzrock R. Clin. Cancer Res. 2008, 14 (14), 4491-9.
8) Pal A, Sung B, Bhanu Prasad BA, Schuber PT Jr, Prasad S, Aggarwal BB, Bornmann WG. Bioorg. Med. Chem. 2014, 22 (1), 435-9.
9) Hassaninasab A, Hashimoto Y, Tomita-Yokotani K, Kobayashi M. Proc. Natl. Acad. Sci. USA 2011, 108 (16), 6615-20.
10) Kang N, Wang MM, Wang YH, Zhang ZN, Cao HR, Lv YH, Yang Y, Fan PH, Qiu F, Gao XM. Food Chem. Toxicol. 2014, 67, 193-200.
11) Lee SL, Huang WJ, Lin WW, Lee SS, ChenCH. Bioorg. Med. Chem. 2005, 13 (22), 6175-81.
12) Calabrese V, Guagliano E, Sapienza M, Panebianco M, Calafato S, Puleo E, Pennisi G, Mancuso C, Butterfield DA, Stella AG. Neurochem. Res. 2007, 32 (4-5), 757-73.
13) Chen CY, Yang WL, Kuo SY. Nat. Prod.Commun. 2011, 6 (11), 1671-2.
14) Srimuangwong K, Tocharus C, Yoysungnoen Chintana P, Suksamrarn A, Tocharus J. World J. Gastroenterol. 2012, 18 (19), 2383-9.
15) Feng Li, Viriya Nitteranon, Xiaozhen Tang, Jin Liang, Guodong Zhang, Kirk L. Parkin, Qiuhui Hu. Food Chemistry 2012, 135 (2), 332-7.
16) Dong HP, Yang RC, Chunag IC, Huang LJ, Li HT, Chen HL, Chen CY. Nat. Prod. Commun. 2012, 7 (7), 883-4.
17) Majeed M. The Age of Biotransformation, 2015; Personal communication.
18) Funk JL, Oyarzo JN, Frye JB, Chen G, Lantz RC, Jolad SD, Sólyom AM, Timmermann BN. J. Nat. Prod. 2006, 69 (3), 351-5.
19) Gupta NK, Dixit VK. J. Pharm. Sci. 2011, 100 (5), 1987-95.
20) Rak K, Rader DJ. Nature News and Views 2011, 472, 40-1.
21) Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Nature 2011, 472, 57-63.
22) Sasaki H, Sunagawa Y, Takahashi K, Imaizumi A, Fukuda H, Hashimoto T, Wada H, Katanasaka Y, Kakeya H, Fujita M, Hasegawa K, Morimoto T. Biol. Pharm. Bull. 2011, 34 (5), 660-5.
23) Cox KH, Pipingas A, Scholey AB. J Psychopharmacol. 2014; pii: 0269881114552744.
24) Drzewiecka A, Kwiatkowska K, Sobota A. Acta Biochim. Pol. 1999, 46 (1), 107-16.
25) Gerloczy A, Hoshino T, Pitha J. J. Pharm. Sci. 1994, 83 (2), 193-6.
26) Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Planta Med. 1998, 64 (4), 353-6.
27) Singh J, Dubey RK, Atal CK. J. Pharmacol. Exp. Ther. 1986, 236 (2), 488-93.
28) Volak LP, Hanley MJ, Masse G, Hazarika S, Harmatz JS, Badmaev V, Majeed M, Greenblatt DJ, Court MH. Br. J. of Clin. Pharm. 2013, 7592, 450-62.
29) Ranjan AP, Mukerjee A, Helson L, Vishwanatha JK. J. Nanobiotechnology 2013, 11, 40; doi: 10.1186/1477-3155-11-40.
30) Helson L, Bolger G, Majeed M, Vcelar B, Pucaj K, Matabudul D. Anticancer Res. 2012, 3 (10), 4365-70.
Recommended Curcumin Product




